1
2
3
4
5
6
7
HOME
Experiment 2: Method/Instrumentation
Principles of UV Spectroscopy:
Absorption spectroscopy relates the amount and type of radiant energy absorbed by a material
to its structure, concentration and identity. Electromagnetic radiation is usually viewed as
a stream of discrete packets of energy called photons. According to quantum mechanics, atoms
and molecules can occupy a limited series of energy states that correspond to distinct energy
levels. To be absorbed, the energy of the incoming radiation must exactly match the difference
between two of the substancešs energy levels. Since atoms and molecules have mostly unique energy
configurations (electron configurations, vibrational/rotational modes etc.), detection by absorption
spectroscopy can be made specific. Furthermore, since absorption is proportional to the
concentration of sample analyte, spectroscopy can be used to quantify the amount of material
present in an unknown.
UV-Vis spectroscopy is based on the selective absorption of electromagnetic radiation in the
180-780 nm wavelength range. UV-Vis radiation has sufficient energy to cause transitions in
bonding electrons (as opposed to atomic innershell or valence electrons) and thus, is correlated
best with the behavior of bonds and functional groups in the analyte. Functional groups having
electrons with relatively low excitation energies absorb strongly in the UV-Vis region, and are
called chromophores.
Absorption in the UV-Vis range is due to electrons participating directly in bond formation or to
unshared, outer electrons that are localized about electronegative atoms such at oxygen, the halogens,
sulfur and nitrogen (8). Either type of electron can be promoted to a higher energy molecular orbital.
Molecular orbitals result from the overlap of atomic electron orbitals during bond formation between
two atoms. The valence electrons of each atom are arranged in molecular orbitals in a way that
minimizes repulsive coulombic forces between neighboring electrons, and between the positively
charged nuclei. When two atomic orbitals combine they form both a low energy bonding molecular
orbital, and a high energy antibonding molecular orbital (designated by an *). Moreover, s and
p atomic orbitals overlap in different ways. The head-on overlap of two s or two p atomic orbitals
is called a sigma bond and the parallel overlap of two p atomic orbitals is called a pi
bond. Single bonds are usually sigma bonds, where as double bonds contain one sigma bond and
one pi bond. Thus, the net number of molecular orbitals for a double bond are sigma, sigma*, pi, and pi*.
Each of these molecular orbitals represents a different energy level as shown in Figure 1 (8).
FIGURE 1
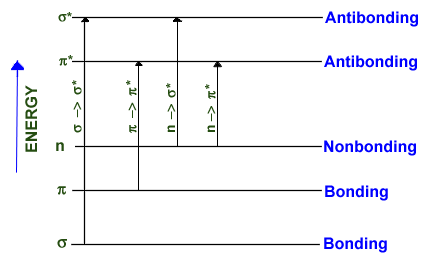
Each atom in a organic molecule in its ground state (low energy state) will contain bonding electrons
in sigma and pi molecular orbitals, and outer, nonbonding unshared electrons (designated by n). The energy
relationship between electrons in bonding, antibonding and nonbonding orbitals is: bonding sigma*, n --> sigma*, n -->pi* and pi --> pi*. Transitions among
these orbitals are brought upon by radiation with energy equaling exactly the energy difference between
the specific orbitals.
Transitions to sigma* orbitals (sigma --> sigma*, n --> sigma*) requires high energy radiation of wavelengths less than
200 nm which usually is not encompassed in a UV-Vis spectral analysis. Vacuum UV or X-ray radiation
is necessary to cause sigma --> sigma* transitions. Molecules such as water and methanol absorb maximally at
167-184 nm due to n --> sigma* transitions.
Transitions to a pi* orbital requires the presence of an unsaturated functional group (chromophore) to
supply the pi* orbitals. Radiation in the 200-700 nm range brings about these transitions making molecules
with chromophores convenient for analysis using a UV-Vis spectrophotometer. Furthermore, n electrons
are very sensitive to the stabilizing effect of polar solvents making the solvent another factor in
identification and interpretation. Outer nonbonding electrons can form extensive, stabilizing hydrogen
bonds with water and alcohols, whereas inner p electrons are unaffected by solvent choice (8).
Organic molecules with conjugated double bonds, carbonyl groups, carboxyl groups, and nitro groups are
the best absorbers in the UV-Vis range. Each functional group has a wavelength associated with an
absorption maximum that can be used for qualitative identification in an unknown sample. The maxima
are strongly affected by substituant groups. For instance, aromatic hydrocarbons have a distinct absorption band
at around 280 nm due to a pi --> pi* transition.
Beer's Law:
UV spectroscopy can be used to quantify the amount of an absorbing material present. The absorbance
A of a solution is defined as:
(1) A = -log10T = log10(Po/P)
Where T, the transmittance, is the fraction of the incident radiation that is transmitted by the
solution, P0 is the power of the incoming radiation and P is the power of the attenuated radiation that
has passed through the sample. Absorption is proportional to the path length b through a sample solution
and the concentration c of an absorbing species according to Beer's Law.
(2) A = ebc
The constant of proportionality in Beer's Law, e, is the molar absorptivity, and is expressed in units
L mol-1 cm-1 when c is expressed in molarity and b in cm. To avoid reflection and scattering error by
the sample holding cell and solvent, the power of the radiation beam transmitted by the solution is compared
to the power of a beam transmitted by an identical cell containing only the solvent. Thus, Beer's Law is
usually written as:
(3) A = log10(Psolvent / Psolution)
Beer's Law can be derived by considering that the fraction of the incoming beam absorbed is proportional to
the probability of capture by the analyte in solution. The probability of capture is proportional to the
surface area of absorbing particles which can then be related to the number, and concentration of particles.
Using Beer's Law, the absorbance of a sample can be related to its concentration. Absorbances are additive,
and Beer's Law can be used to determine the concentrations of a mixture of species, as long as they are not
interacting. Beer's Law is limited in that it applies only to dilute solutions. In concentrated solutions
(>0.01 M) particles interact altering the analyst's ability to absorb a certain wavelength of radiation which
causes non linear deviations from Beer's Law. Changes in the refractive index of the solution, and chemical
reaction will also cause error in applying Beer's Law to a sample. Finally, Beer's Law is only valid for
monochromatic radiation. Thus, the most accurate result will be obtained by using a source able to produce
intense radiation at a single wavelength.
UV-Visible Spectrophotometers:
Spectrophotometers are made up of stable source of radiant energy, a transparent sample container, a device
for isolating specific wavelength, a radiation detector which converts transmitted radiation to a usable
signal, and a signal processor and readout. Each of these components will be discussed in reference to
the Cary 1-E UV-Vis Spectrophotometer used in this experiment.

Students measure sample spectra on a Cary UV-Vis Spectrophotometer
Sources
A radiation source for spectroscopy must generate a beam with sufficient power, wavelength range and
stability for detectable and reproducible results. Many UV-Vis spectrophotometers such as the Cary 1-E,
use a deuterium lamp for the UV range and switch to a tungsten filament lamp at 350 nm for the visible
range. The electrical excitation of deuterium at low pressure results in a continuous spectrum of
emitted radiation from 160 nm to the beginning of the visible (375 nm). An arc is formed between a
heated, oxide-coated filament and a metal electrode. When about 40 Volts is applied to the heated
filament, a direct current is produced resulting in an intense ball of radiation.
At around 350 nm the Cary 1-E switches its radiation source to a tungsten filament.
The radiant energy emitted from a heated tungsten filament approaches that of a black body and
thus, is temperature dependent. The operating temperature is usually 2870 K which results in radiation
in the 350-2500 nm range. The lower limit is due to absorption at 350 nm by the glass container
housing the filament.
The problem of source stability can be resolved by using a duel beam system where radiation is
directed at a sample and a solvent reference at the same time. Fluctuations in the source will
be canceled. The Cary 1-E utilizes a duel beam system.
Sample containers
Sample containers (cells or cuvettes) must be constructed of a material that is transparent to
radiation in the wavelength range of interest. Containers of quartz or fused silica are necessary
in the UV range, and can be used into 700-3000 nm infra-red region. Glass and plastic can be used
in the visible region as well. To minimize reflection cuvettes should have windows that are perfectly
normal to the direction of the beam. Quartz cuvettes with a 1 cm path length were used with the Cary 1-E.
Wavelength Selector, Radiation Detector and Signal Processor
A schematic of the Cary 1-E inner workings is shown in Figure 2. Table 1 summarizes the components
of the Cary 1-E.
FIGURE 2
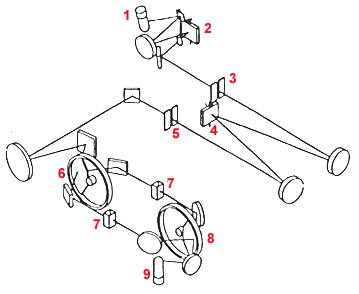
TABLE 1
| Number | Label | Description |
| 1 | D2/QI lamp | Radiation Source |
| 2 | Grating 1 | A colored glass filter wheel that isolates a
rough region of wavelengths around the desired wavelength. |
| 3 | Entrance slit | Isolates a thin beam of radiation coming from the
filter wheel, limits the range of incidence angles. |
| 4 | Grating 2 | A holographic grating that disperses the radiation allowing a
very precise selection of wavelengths. The grating is 30 x 35 mm in size and can move
between wavelengths at 3000 nm/minute. |
| 5 | Exit slit | Radiation from the holographic grating is reflected by a
mirror to the exit slit. The desired wavelength is selected by rotating the grating
relative to the exit slit. Radiation of undesired wavelengths is absorbed by black matte
walls surrounding the grating. |
| 6 | Chopper 1 | Radiation from the exit slit is directed by mirrors at the
first chopper. The chopper consists of three sections as shown in Figure 4 and rotates at
30 Hz. The chopper has a cut-out section as shown, which allows radiation to pass through
the chopper to a mirror which directs it to the sample cell. The next section on the chopper
is a mirrored which reflects the beam to another mirror, and then to the reference solvent
cell. Light hitting the black section is absorbed. Thus, the beam hits the sample and
reference almost instantaneously. |
| 7 | Sample position | The beam is focused on the center of the sample compartment
to allow maximum light throughput and reduce noise. |
| 8 | Chopper 2 | The second chopper ensures that the sample beam and reference
beam hit the phototube detector at the same position and angle since the detector is sensitive
to these quantities. The mirrored section reflects the sample beam to another mirror, and then
to the phototube detector. The chopper then rotates to the cut-out section which allows the
reference beam through to the detector. The solid section of the chopper absorbs all radiation
and blocks the beam from hitting the phototube detector. |
| 9 | Phototube | The phototube detector first corrects for any residual signal in
the detector by zeroing itself during the moment when the black section of the choppers block
the light beam. Next the sample and reference beams are sequentially measured. The transmission
is determined by:
T = (sample - dark signal) / (reference - dark signal)
The transmission signal is sent to a computer where it is converted to absorbance. The result is
show on the CRT. |
Interferences:
The most obvious source of error in correlating UV-Vis absorption with DOC is from nonabsorbing
organic material present in the sample. Simple sugars, aliphatic acids, alcohols and amino acids,
such as glycine, do not absorb in the UV range which results in an underestimation of the DOC (4).
However, these compounds are those most likely to be consumed by microbes as food, and are not expected
to be present in natural water samples from rivers and lakes at high concentrations.
Other possible sources of interference with the desired the UV-Vis spectra can come from particulate
matter, nitrate/nitrite, chlorine, and/or ozone in the sample. Nitrate, a nutrient ubiquitous to
natural water, absorbs at 313 nm due to a n-->pi* transition. Nitrite also absorbs at 280 nm and
360 nm, but is not usually found in natural water or treated waste water. The nitrate used to lower
the pH of our samples may have interfered with the absorption spectra by giving a higher absorbance
than for sample alone.
The presence of oxidants such as chlorine or ozone in the samples can cause a reduction in the
absorbance at 254 nm without a corresponding reduction in DOC. Small amounts of these compounds
can partially oxidize some of the NOM to CO2. In the process, less aromatic, unabsorbing by-products
are formed. For example, the by-products of chlorine addition to natural water can result in di- and
tri- chloroacetic acid (5). It also has been shown in laboratory experiments that ozone addition to
fulvic acid solutions causes a reduction in absorbance at 254 nm (5).
Particulate matter can affect the UV-Vis absorbance by scattering incoming radiation. Scattering
by suspended material in the sample increases with particle size with maxim scattering, for a
given weight of sample, occurring at a particle size equal to the wavelength of incoming radiation.
Therefore, UV radiation is more sensitive to turbidity as it is scattered by smaller particles
(<500 nm diameter) than is radiation of longer wavelength. Although the natural water sample in this study was unfiltered, little particulate organic carbon was present, and no particulate material was visible. Dobbs et. al found that unfiltered, clear samples of natural water were adequate to preform error-free concentration studies with UV-Vis absorbance (4).
Calibration Procedure:
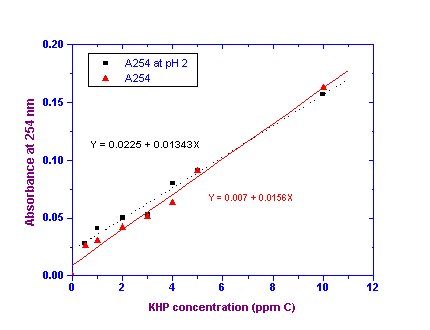
A standard curve relating A254 to the concentration of KHP in ppm C was plotted as shown
in Figure 3. A typical regression analysis was performed on the data resulting in the linear
curve fit and equations as shown. The relevant parameters are displayed in the RESULTS section.
All errors are reported at the 95% confidence level.
FIGURE 3
Results



